Calcium Carbonate Standard , (1mL = 1mg CaCO3 = 0.4mg Ca), for Hardness, Certified, 1.000 0.010mg CaCO3/mL, LabChem, Quantity: Each of 1 | Fisher Scientific

Alkalinity P and T to test mixtures of hydroxide and carbonates, 1 drop = 10 ppm as CaCO3/10ml (CIP) - WET INTERNATIONAL, INC.

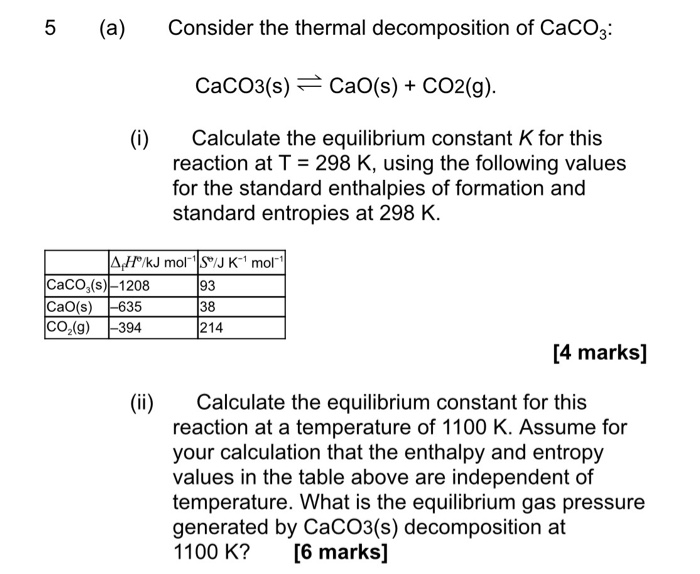
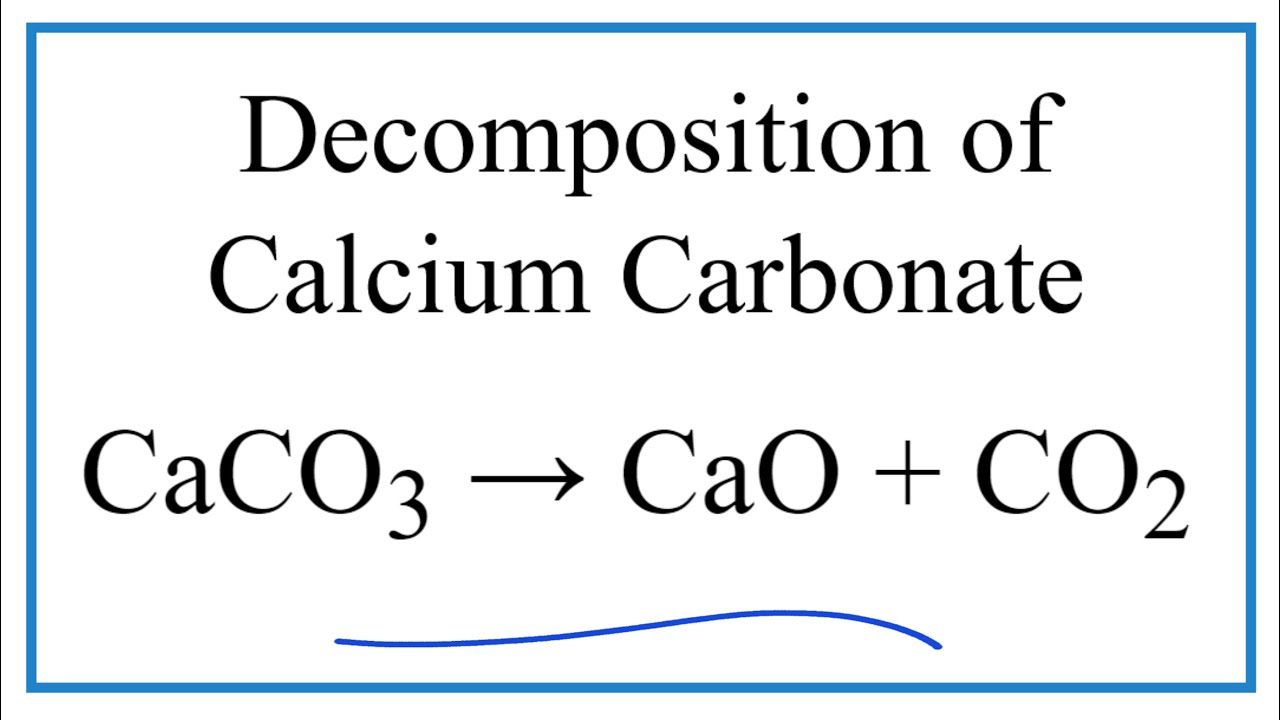
In the preparation of CaO from CaCO3 using the equilibrium CaCO3(s) CaO(s) + CO2(g) Kp is expressed as: logKp = 7.282 - 8500T for complete decomposition of CaCO3 the temperature in celsius

SOLVED: Given the following reactions: CaCO3 (s) â†' CaO (s) + CO2 (g) ΔH° = 178.1 kJ C (s, graphite) + O2 (g) â†' CO2 (g) ΔH° = -393.5 kJ What is

Polymers | Free Full-Text | Research on Properties of PBAT/CaCO3 Composite Films Modified with Titanate Coupling Agent

Nano CaCO3 for Offset Ink (O/DD/T-DD series) - China Calcium Carbonate, Nano CaCO3 | Made-in-China.com
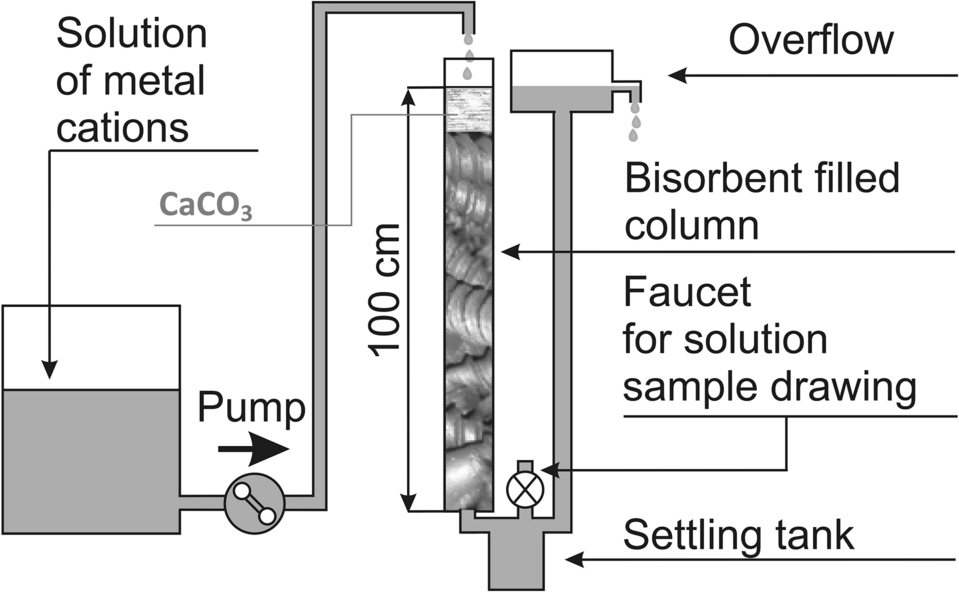
Role of calcium carbonate in the process of heavy metal biosorption from solutions: synergy of metal removal mechanisms | Scientific Reports

Green Inhibitor Performance against CaCO3 Scaling: Rate-Modeling Aided Test Procedure | Crystal Growth & Design

Nano CaCO3 for Offset Ink (O/DD/T-DD series) - China Calcium Carbonate, Nano CaCO3 | Made-in-China.com
![Adsorption of phenol over bio-based silica/calcium carbonate (CS-SiO2/CaCO3) nanocomposite synthesized from waste eggshells and rice husks [PeerJ] Adsorption of phenol over bio-based silica/calcium carbonate (CS-SiO2/CaCO3) nanocomposite synthesized from waste eggshells and rice husks [PeerJ]](https://dfzljdn9uc3pi.cloudfront.net/2021/pchem-17/1/fig-1-full.png)
Adsorption of phenol over bio-based silica/calcium carbonate (CS-SiO2/CaCO3) nanocomposite synthesized from waste eggshells and rice husks [PeerJ]
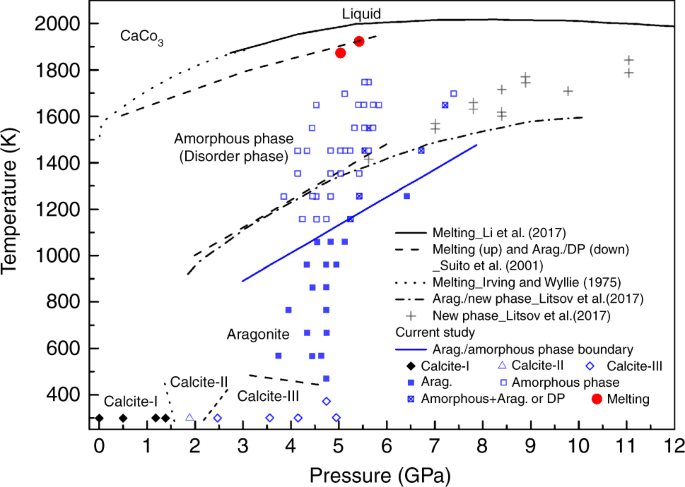
Temperature-induced amorphization in CaCO3 at high pressure and implications for recycled CaCO3 in subduction zones | Nature Communications

![Kannada] CaCO3 decomposes to give CO2 gas according to the equation. Kannada] CaCO3 decomposes to give CO2 gas according to the equation.](https://d10lpgp6xz60nq.cloudfront.net/ss/web-overlay-thumb/8538345.webp)