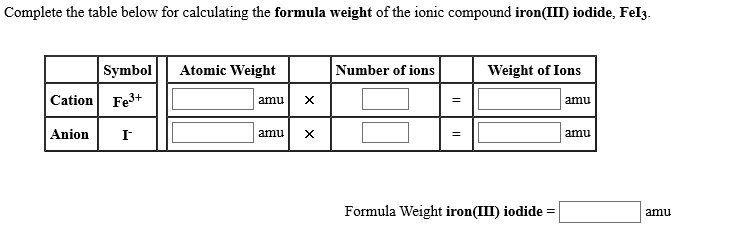
SOLVED: Consider the reaction between iron (III) sulfate and barium iodide. If barium iodide is present in excess, determine the amount of iron (III) sulfate needed to produce 5.84×10^24 molecules of iron (

SOLVED: Does a reaction occur when aqueous solutions of iron(III) iodide and sodium carbonate are combined? yes/no If a reaction does occur, write the net ionic equation.

Iron transition metal Chemistry iron(II) Fe2+ iron(III) Fe3+ complexes ions ligand substitution redox chemical reactions principal oxidation states +2 +3 extraction iron analysis by titration GCE AS A2 IB A level inorganic
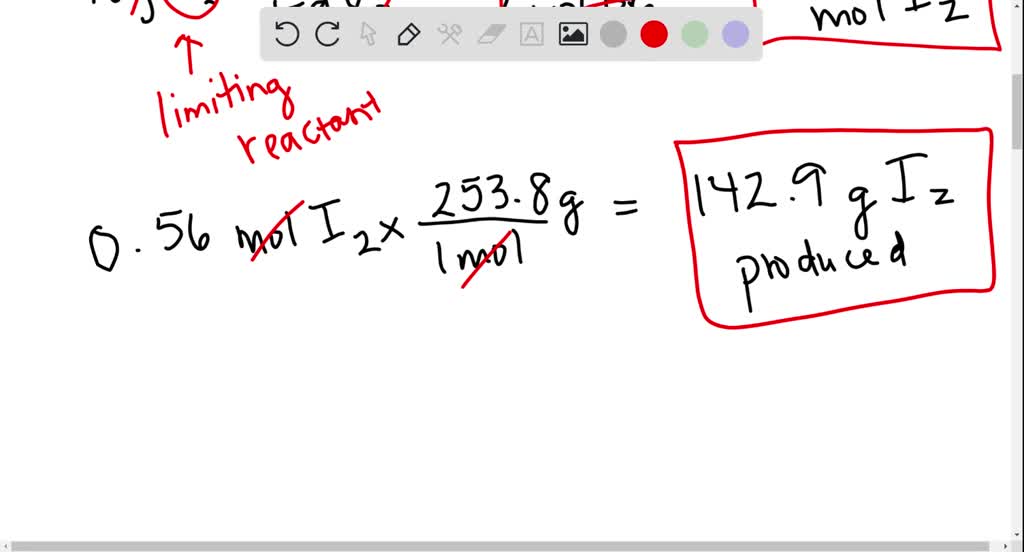
SOLVED: Iron (III) iodide reacts with bromine to produce iodine and iron(III) bromide. 2 FeI3 + 3 Br2 â†' 3 I2 + 2 FeBr3 a. If 218 g of iron (III) iodide

Profile of iodine liberation by oxidizing iodide ions with iron(III).... | Download Scientific Diagram